This is the first in a series of essays based on the grand rounds presentation I delivered as a chief resident in general surgery this year.
Our frame story begins in 1847 with Ignaz Semmelweis and unacceptable maternal mortality rates at the Vienna Hospital.
Semmelweis observed medical students conducting dissection on cadavers in the pathologic anatomy labs between childbirths, and hypothesized an association between some sort of contamination event occurring in the anatomy labs and the epidemic of maternal mortality. He suggested that the elimination of this contamination event through antisepsis would lead to a dramatic reduction of this mortality; and indeed the implementation of mandatory chlorinated handwashing eliminated the Puerperal fever epidemic that had plagued the Vienna hospital for nearly half a century.
Semmelweis's hypothesis — intra-operative contamination events give rise to post-operative infections — would become the first principle upon which the next century of research around surgical infection would be built.
20 years after Semmelweis, in 1867, came Lister and carbolic acid.
Surgeons were desperate for a way to avoid infections, and aseptic principles were rapidly adopted. Below are two paintings of surgeons at work in Philadelphia in the late 1800s.
In 1875, Samuel Gross and his assistants are wearing their street clothes. No one is wearing gloves. Surgical instruments are stored in what is essentially a toolbox.
Twelve years later, in 1889, David Agnew is wearing surgical whites. He and his resident are wearing gloves. The operative field is isolated with drapes. Instruments are in a sterilization tray.
(Some things never change: in both paintings, medical students are asleep in the audience.)
After clean OR and handwashing and sterile instruments came antibiotic prophylaxis and fancier and fancier sterilization methods and laminar airflow HEPA-filtered operating rooms and double-gloving and all the other accumulated effort of the surgical discipline over the past century and a half to eliminate intra-operative contamination events.
But what do we observe?
Surgical infections keep happening!
With the advent of modern murine models, perhaps we can try and experimentally produce surgical infections in the lab so that we can study them under necessary and sufficient conditions.
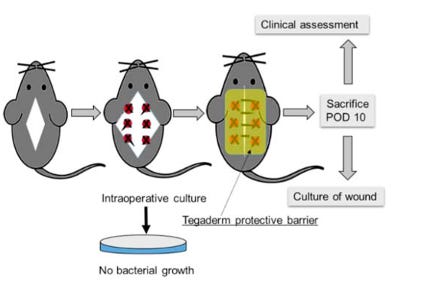
Let's consider an experimental model1 in which mice undergo a surgery consisting of an incision and instrumentation at 6 sites along the muscles of the back.
At each site, electrocautery is applied and a suture ligature is placed. Cultures are obtained at the time of surgery, to confirm the surgical tissues are sterile up until this point.
The wounds are then directly contaminated with bacterial cultures intra-operatively, and again and again for several days. On day 10 after surgery, the mice are sacrificed so the wounds can be assessed using this clinical wound infection score:
Scores range from 0 to 8 and characterize the type of discharge, the degree of dehiscence, the presence of an abscess, and culture growth.
What do we observe?
Despite direct contamination of the surgical wounds with bacterial cultures, clinically significant infections develop in only 20% of the mice. This sort of yield isn't nearly sufficient for experimental testing — 80% of our specimens would never even develop clinically significant infections to be experimented on.
It is only when we replace standard mouse chow (standard diet, SD) with a low-fiber, high-fat, high-carb western diet (WD) that we begin to reliably produce surgical site infections in this experimental model.
What if the bacteria causing these surgical infections are coming from the gut microbiome instead?
We can propose the following experiment, in which mice are not directly contaminated, but instead undergo a sterile surgery. There are four groups, western diet vs standard diet and peri-operative antibiotics versus none.
And what do we observe?
Rather than the introduction of antibiotics reducing infectious rates in the WD group back down to the rates for the SD group, we find that giving antibiotics worsens both the clinical wound infection score as well as increases the rate of polymicrobial infection in both the WD and SD groups.
What could possibly be going on here? We are sterilizing the animal with IV antibiotics at the time of surgery, and this is producing more infections? Where are these bacteria coming from?
Again, we can test this experimentally2, and with fluorescence imaging we can demonstrate that the causative bacteria originate at mucosal surfaces like the gums and the gut lining.
Image D on the left is a cecum of a mouse that did not receive antibiotics. Image C shows the cecum of a mouse that did receive antibiotics, which has now become colonized by fluorescent MRSA.
We can watch under FISH imaging as this MRSA is silently taken up by neutrophils at the mucosal border through phosphate-seeking chemotaxis behavior that is conserved by (effectively) all bacteria. These trojan-horse neutrophils can later travel to a remote site of inflammation — like the surgical wound in image B on the right — and release their infectious payload.
This is the Trojan horse hypothesis, in which the intestinal epithelium becomes progressively colonized by phosphate-seeking bacteria, which are silently transported to remote sites of inflammation within neutrophils, where they eventually release their infectious payload and produce surgical site infections far remote from the site of true "contamination" at the mucosal barrier.
What can we learn from this?
Well, at some point between Semmelweis and single use scrubs, the causal inference got out of hand.
What Semmelweis observed in 1847 was obviously real, and the advent of germ theory and antibiosis and aseptic surgery has saved countless lives.
At the same time, the discoveries that initially led to progress eventually became assumptions that limit our ability to see the error space and continue to produce useful improvements.
Hyoju, Sanjiv K. MD*; Keskey, Robert MD*; Castillo, Gerardo BS†; Machutta, Kaylie BS‡; Zaborin, Alexander PhD*; Zaborina, Olga PhD*; Alverdy, John C. MD, FACS*. A Novel Nonantibiotic Gut-directed Strategy to Prevent Surgical Site Infections. Annals of Surgery 276(3):p 472-481, September 2022. | DOI: 10.1097/SLA.0000000000005547
Krezalek, Monika A. MD; Hyoju, Sanjiv MD; Zaborin, Alexander PhD; Okafor, Emeka MS; Chandrasekar, Laxmi MD; Bindokas, Vitas PhD; Guyton, Kristina MD; Montgomery, Christopher P. MD; Daum, Robert S. MD; Zaborina, Olga PhD; Boyle-Vavra, Susan PhD; Alverdy, John C. MD, FACS. Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Annals of Surgery 267(4):p 749-758, April 2018. | DOI: 10.1097/SLA.0000000000002173